Uplizna (inebilizumab-cdon) for neuromyelitis optica
Last updated May 29, 2024, by Marisa Wexler, MS

What is Uplizna for neuromyelitis optica?
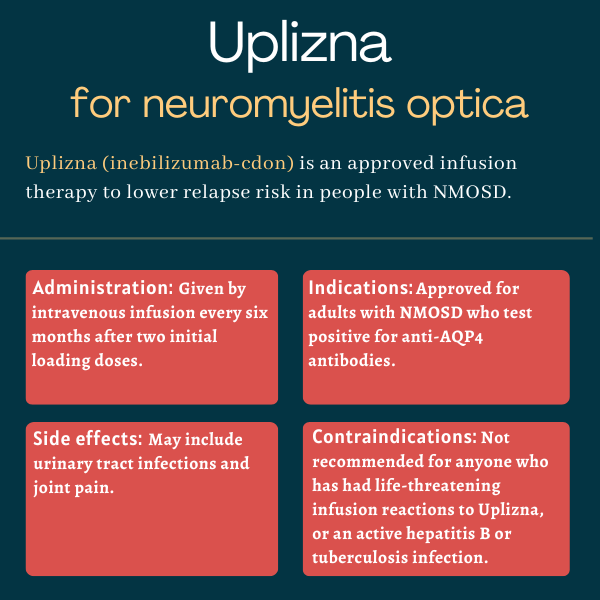
Uplizna (inebilizumab-cdon) is an infusion therapy approved for adults with neuromyelitis optica spectrum disorder (NMOSD) who test positive for antibodies against the aquaporin-4 (AQP4) protein — the most common target of NMOSD-driving self-reactive antibodies.
Administered intravenously, or directly into the bloodstream, the therapy is designed to reduce the risk of NMOSD relapses by targeting the immune cells responsible for producing antibodies, including those driving the autoimmune disease.
Uplizna was initially developed by Viela Bio, but after a series of acquisitions, it is now marketed by Amgen.
Therapy snapshot
| Brand name: | Uplizna |
| Chemical name: | Inebilizumab-cdon |
| Usage: | Used to lower the risk of relapses in adults with NMOSD |
| Administration: | Intravenous infusion |
How does Uplizna work?
NMOSD is an autoimmune disorder in which the immune system mistakenly attacks the body’s own healthy cells, leading to damaging inflammation mainly in the optic nerve and spinal cord. The optic nerve transmits signals between the eyes and the brain.
This autoimmune attack is spearheaded by abnormal, self-reactive antibodies that most commonly target AQP4, a protein highly present at the surface of neuron-supporting cells called astrocytes.
Uplizna is an antibody-based therapy that binds to CD19, a cell surface protein specifically found in immune B-cells. These B-cells are responsible for producing antibodies, not only those used to fight invaders, but also the self-reactive antibodies that drive NMOSD.
By targeting CD19, Uplizna results in a depletion of B-cells at nearly all maturation stages, thereby lowering levels of anti-AQP4 antibodies and ultimately limiting the autoimmune attacks that drive NMOSD.
As such, the therapy is expected to ease NMOSD symptoms and reduce the risk of relapses, which are linked to increasing disability.
Who can take Uplizna?
Uplizna was approved by the U.S. Food and Drug Administration (FDA) in June 2020 for adults with NMOSD who test positive for antibodies against AQP4. The decision made Uplizna the second approved therapy for this patient population and the first to involve administration only twice a year, after initial dosing.
Uplizna has received approvals for a similar indication in many other markets, including Europe and Canada.
Who should not take Uplizna?
Uplizna is not recommended for anyone with:
- a previous life-threatening reaction to infusion of the therapy
- active hepatitis B, a viral infection that mainly affects the liver
- an active or untreated latent tuberculosis infection, a bacterial infection that usually attacks the lungs.
The therapy should also not be given to patients with any other active infection, and treatment should be delayed until the infection is resolved.
How is Uplizna administered in neuromyelitis optica?
Uplizna, available in single-dose vials containing 100 mg of the medication, is administered via an infusion into the bloodstream at a recommended dose of 300 mg. The therapy is prepared and given by an experienced healthcare provider.
The first two infusions are given two weeks apart, and subsequent infusions are given once every six months. Each infusion lasts about 90 minutes, and patients need to be monitored for at least one hour after the infusion ends for signs or symptoms of infusion-related reactions.
Before each infusion, patients should also receive three types of pre-medication to help prevent or reduce the severity of infusion reactions:
- a corticosteroid, such as methylprednisolone, administered directly into the bloodstream 30 minutes before the infusion
- an oral antihistamine such as diphenhydramine, given 30 to 60 minutes before dosing
- an oral anti-fever medication such as acetaminophen (sold as Tylenol, among others), given 30 to 60 minutes before the infusion.
Uplizna in clinical trials
Uplizna’s approvals were based mainly on results from a Phase 2/3 clinical trial called N-MOmentum (NCT02200770).
N-MOmentum
The global N-MOmentum study enrolled 230 adults with NMOSD — 213 of whom were positive for antibodies against AQP4 — who had experienced at least one NMOSD relapse requiring treatment in the previous year or two relapses requiring rescue therapy in the previous two years.
In the trial, about three-quarters of the participants (75.7%) were treated with Uplizna at the now-approved dosing schedule, while the remaining quarter (24.3%) were given a placebo.
Participants were followed for 197 days (about six months) or until they experienced a disease relapse, whichever came first. The study’s main goal was to determine whether Uplizna treatment extended the time to experiencing a relapse relative to the placebo.
Results showed Uplizna treatment significantly reduced the risk of relapse, by about 77.3%, among patients who were positive for anti-AQP4 antibodies. In this group of patients, a relapse was experienced by 11.2% on Uplizna compared with 42.3% given a placebo.
Uplizna also significantly reduced the risk of disability worsening in AQP4-positive patients, as assessed with the Expanded Disability Status Scale (EDSS), meeting one of the trial’s secondary goals.
Specifically, Uplizna-treated patients had a 62.5% lower risk of three-month confirmed disability worsening compared with those on a placebo. These benefits were independent of pre-treatment EDSS score, disease duration, and number of previous attacks. In addition, patients treated with Uplizna were 66.3% more likely to report lessened disability on the modified Rankin Scale than those in the placebo group.
Patients on Uplizna also had significantly fewer active lesions on MRI and less NMOSD-related hospitalizations.
Long-term data
Participants who completed the trial’s placebo-controlled portion without experiencing a relapse had the option to enroll in its open-label extension, wherein all were treated with Uplizna and monitored for long-term outcomes.
Findings covering at least four years of Uplizna treatment showed more than 80% of AQP4-positive patients remained free of relapses during that period, regardless of whether or not they were originally assigned to the therapy in the main trial.
Most patients who relapsed did so in the first year of treatment. After one year, 92% remained relapse-free. In addition, disability scores remained generally stable throughout the more than four years of treatment.
Further analysis of N-Momentum’s four-year data indicated Uplizna also eased pain and reduced the likelihood of new disease-related damage in both the spinal cord and optic nerves.
Among the 17 N-MOmentum participants who were negative for anti-AQP4 antibodies, there was no evidence that Uplizna was beneficial in the study’s placebo-controlled part. Long-term data have hinted at possible benefits for some of these patients, but there isn’t enough data to say confidently whether Uplizna benefits NMOSD patients without anti-AQP4 antibodies.
Ongoing trials
Patients completing at least two years of Uplizna treatment in N-Momentum’s extension portion were given the choice to enroll in an open-label, long-term Phase 4 trial (NCT06180278), in which they continue the treatment for up to 3.5 additional years.
The study, also open to NMOSD patients newly starting on Uplizna, will assess the therapy’s long-term safety and efficacy in up to 30 participants. It is expected to conclude in 2028.
An international Phase 2 trial (NCT05549258) is also evaluating Uplizna’s safety, pharmacological properties, and efficacy in up to 15 children and adolescents, ages 2-17 years, with NMOSD and antibodies against AQP4.
All participants are receiving the therapy for about six months. Efficacy goals include time to first relapse, proportion of relapse-free patients, and annualized relapse rate, as well as changes in EDSS score, vision, and health-related quality of life. The study is set to end in 2027.
Common side effects of Uplizna
The most common side effects of Uplizna reported in clinical trials included urinary tract infections and joint pain.
Infusion reactions
Uplizna can cause infusion-related reactions, or side effects that appear during or shortly after the infusion, with symptoms including headache, nausea, sleepiness, shortness of breath, fever, muscle pain, and rash.
In the N-MOmentum trial, about one in 10 patients experienced such a reaction during the first infusion, and reactions were less common (but still could occur) in later infusions. To lower the risk of infusion-related reactions, patients should receive preventive treatment with a corticosteroid, an antihistamine, and an anti-fever drug before each Uplizna infusion.
If a disabling or life-threatening infusion reaction occurs, Uplizna treatment should be immediately and permanently discontinued, and appropriate supportive care should be administered at once. For milder reactions, management may include pausing or slowing the infusion, and/or giving supportive treatment to ease symptoms.
Infections and vaccines
Due to its immunosuppressive effects, Uplizna can increase the risk of new infections or cause existing infections to worsen. This risk of infection may be further heightened if the therapy is given in combination with other immunosuppressive medications.
Before starting on Uplizna, patients should be checked for hepatitis B and tuberculosis — special considerations may be needed for patients with inactive latent infections of either pathogen, while the therapy should not be given to those with active infections.
In fact, the therapy should not be administered to anyone with an active infection of any kind, and a planned infusion should be delayed until the infection is resolved.
There have been reports of progressive multifocal leukoencephalopathy, a rare but potentially life-threatening brain infection, in people given other B-cell-depleting medicines. As such, patients on Uplizna should be monitored for symptoms of this infection, including progressive weakness or clumsiness, vision problems, confusion, and cognitive and personality changes. If such symptoms occur, Uplizna treatment should be stopped and the patient should undergo appropriate diagnostic testing.
The FDA recommends patients receive all recommended vaccines at least four weeks before starting on Uplizna. Vaccines that contain a live or a live attenuated (weakened) virus are not recommended during Uplizna treatment and until B-cells return to normal levels after stopping treatment.
Such vaccines should also not be given to newborns who have been exposed to Uplizna while in the womb until after B-cells have recovered to normal levels. Caution is still advised when administering vaccines not containing a live virus.
Reduced antibody levels
Because Uplizna works by destroying B-cells, the therapy can cause a progressive and prolonged drop in antibody levels, which can contribute to an increased risk of infections. Levels of antibodies, also called immunoglobulins, should be monitored regularly while on Uplizna and after discontinuation until B-cell counts return to normal.
Uplizna may be discontinued in patients with low antibody counts who experience frequent infections, as well as in those with prolonged antibody deficiency that needs treatment with intravenous immunoglobulin, or into-the-vein administration of healthy antibodies.
Use in pregnancy and breastfeeding
Based on animal data, Uplizna may cause harm to a developing fetus if used during pregnancy, and data from people have shown that newborns exposed to Uplizna while in the womb often have abnormally low counts of B-cells and other immune cells.
Due to these risks, Uplizna is not recommended for use in pregnancy, and patients able to become pregnant are advised to use effective contraception while on the therapy and for at least six months after the last dose.
No data are available about whether Uplizna is excreted in human milk, though similar molecules are usually present in breast milk. Patients and providers are advised to carefully weigh the potential benefits and risks of using Uplizna while breastfeeding to make decisions on a case-by-case basis.
Neuromyelitis News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Approved NMOSD therapies better than rituximab at preventing relapses
- Embracing an opportunity for advocacy on Rare Disease Day
- Cognitive issues affect nearly 1 in 3 with NMOSD, study finds
- With AI’s expanded use in healthcare settings, I’m excited for the future
- Pure oxygen therapy may reduce inflammation in NMOSD: Study
Related articles