Ultomiris (ravulizumab-cwvz) for neuromyelitis optica
Last updated March 28, 2024, by Marisa Wexler, MS

What is Ultomiris for neuromyelitis optica?
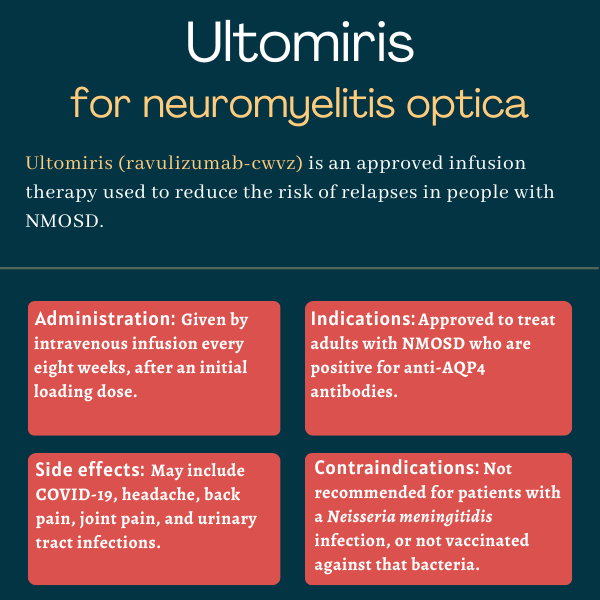
Ultomiris (ravulizumab-cwvz) is an infusion therapy approved for adults with neuromyelitis optica spectrum disorder (NMOSD) who are positive for antibodies against the aquaporin 4 (AQP4) protein. It works to reduce the risk of disease relapses, or flares.
The therapy was developed by Alexion Pharmaceuticals, which was acquired by AstraZeneca in 2021 and is now known as Alexion, AstraZeneca Rare Disease.
Ultomiris is widely approved for disorders sharing some underlying immune-related mechanisms with NMOSD, including generalized myasthenia gravis, atypical hemolytic uremic syndrome, and paroxysmal nocturnal hemoglobinuria.
Therapy snapshot
| Brand name: | Ultomiris |
| Chemical name: | Ravulizumab-cwvz |
| Usage: | Used to treat adults with NMOSD; can reduce disease relapses |
| Administration: | Intravenous infusion |
How does Ultomiris work?
In NMOSD, the immune system produces self-reactive antibodies that wrongly attack and damage healthy cells in the nervous system. The most common target of these antibodies is AQP4, a water channel protein found at the surface of neuron-supporting cells called astrocytes.
The complement cascade, a set of blood proteins that are part of the immune system’s first line of defense, plays a central role in driving the autoimmune attack.
When antibodies such as those against AQP4 bind to their target, they can trigger the activation of the complement cascade, which works like a chain reaction. The first activated complement protein activates the next, which activates the next, and so on, ultimately driving a powerful inflammatory reaction.
One of the later steps in complement activation is the cleavage, or split, of the terminal complement component 5 (C5) into two subunits — C5a and C5b — that go on to promote further inflammation. In NMOSD patients positive for anti-AQP4 antibodies, C5a and C5b contribute to inflammation and astrocyte destruction.
Ultomiris is an antibody-based therapy designed to bind to C5 and prevent this cleavage, thereby suppressing the activation of the complement cascade that helps drive autoimmune damage in NMOSD. By doing so, the therapy is expected to help control symptoms and reduce the risk of disease relapses, which are associated with disability worsening.
The medication works in essentially the same way as Soliris (eculizumab), an older treatment approved for NMOSD patients with anti-AQP4 antibodies. Both Ultomiris and Soliris were developed by Alexion.
The newer therapy was created by introducing a small change to Soliris’ chemical structure. This change makes Ultomiris more stable and long-acting in the body than Soliris, allowing for less frequent dosing. Maintenance doses of Ultomiris are given every eight weeks, compared with Soliris, which is administered every other week.
Who can take Ultomiris?
Ultomiris was approved by the U.S. Food and Drug Administration (FDA) in March 2024 for treating adults with NMOSD who test positive for anti-AQP4 antibodies. The decision made Ultomiris the first long-acting C5 inhibitor to be cleared in the country for NMOSD.
The therapy also is approved for this indication in other countries and regions, including Canada, Japan, and the European Union.
Who should not take Ultomiris?
Ultomiris’ prescribing information carries a boxed warning that it increases the risk of potentially life-threatening meningococcal infections — a type of bacterial infection that commonly affects the membranes around the brain and spinal cord — caused by Neisseria meningitidis.
For this reason, the medication is contraindicated, or not to be prescribed, for anyone with an unresolved serious Neisseria meningitidis infection.
Ultomiris also is not recommended for patients who are not vaccinated against this bacteria, unless the risks of delaying treatment outweigh the potential risk of a serious meningococcal infection. In such cases, patients should receive preventive antibiotic treatment before starting Ultomiris.
To reduce the frequency and severity of meningococcal infections, Ultomiris is available in the U.S. only through a restricted access program called Ultomiris REMS — for Risk Evaluation and Mitigation Strategy.
The program is designed to ensure that prescribing physicians and patients are fully informed about this risk and equipped to respond effectively if any signs of such infections become apparent. Ultomiris REMS also ensures that patients receive necessary preventive measures prior to the start of Ultomiris treatment.
How is Ultomiris administered in neuromyelitis optica?
Ultomiris is given via infusions into the bloodstream, administered by a healthcare provider.
When treatment first starts, patients receive an initial infusion called a loading dose, which contains a slightly lower dosage than subsequent maintenance doses. The first maintenance dose is then given two weeks later; additional doses are administered every eight weeks, or about two months, thereafter.
The recommended Ultomiris loading and maintenance doses are determined by a person’s body weight:
- For NMOSD patients weighing 40 kg to less than 60 kg (about 88 to less than 132 pounds), the loading dose is 2,400 mg and maintenance doses are 3,000 mg.
- For those weighing 60 kg to less than 100 kg (132 to less than 220 pounds), the loading dose is 2,700 mg and maintenance doses are 3,300 mg.
- For patients weighing 100 kg (220 pounds) or more, the loading dose is 3,000 mg and maintenance doses are 3,600 mg.
The minimum infusion time varies based on the type of dose (loading versus maintenance), and the person’s body weight and respective dosage. Overall, the infusion time ranges from less than half an hour to close to an hour.
For patients switching from Soliris to the longer-lasting Ultomiris, the loading dose of the new treatment may be given at the time of the next scheduled Soliris dose.
Supplemental doses of Ultomiris might be required for patients treated with plasma exchange or intravenous immunoglobulin. These therapeutic approaches are often used in NMOSD to reduce the levels of disease-driving antibodies in the blood — but they may also lower Ultomiris levels.
Ultomiris in clinical trials
Ultomiris’ approvals for NMOSD were supported mainly by data from a Phase 3 clinical trial called CHAMPION-NMOSD (NCT04201262).
CHAMPION-NMOSD
Launched in December 2019, the CHAMPION-NMOSD study was designed to evaluate Ultomiris’ safety and effectiveness in adults with NMOSD carrying anti-AQP4 antibodies. A total of 58 patients who had experienced at least one relapse in the year prior were enrolled at 36 sites across 11 countries.
Because three therapies already were approved for NMOSD at the time of the study, no participant was given a placebo. Instead, all participants were treated with Ultomiris according to the now-approved dosing regimen for up to 2.5 years; data were compared with those of an external group of 47 patients who received a placebo in the Phase 3 PREVENT trial (NCT01892345) — the study that supported Soliris’ approvals for AQP4-associated NMOSD.
Inclusion criteria were similar between the CHAMPION-NMOSD and PREVENT studies, and patients in the external control group had comparable demographic and clinical features to those given Ultomiris.
CHAMPION-NMOSD’s main goal was to assess the time to first on-trial relapse and associated relapse risk reduction in Ultomiris-treated patients compared with the external placebo group.
Patients given Ultomiris were followed for a median of 17 months, or nearly 1.5 years. Meanwhile, those in the external PREVENT group were followed for a median of about eight months. In both groups, some patients were followed for more than two years.
No confirmed relapses were seen among Ultomiris-treated patients, but the results showed 20 individuals in the external placebo group experienced relapses. This represented a significant drop, by 98.6%, in the risk of relapse with Ultomiris relative to the placebo.
A 97.9% reduction in relapse risk also was found for patients receiving Ultomiris without additional immunosuppressants, when compared with those given a placebo in PREVENT in the absence of such medications.
In addition, all patients treated with Ultomiris were free from relapses at 48 weeks, or nearly one year, versus 63% of those in the external placebo group.
CHAMPION-NMOSD also met two key secondary goals. It showed that Ultomiris-treated patients had significantly fewer relapses per year than would be expected in an NMOSD population. Ultomiris also was associated with a significantly lower chance of clinically important worsening in walking skills — with 3.4% of patients experiencing such motor worsening versus 23.4% of those given a placebo in PREVENT.
Changes in quality of life and overall disability — as assessed with the expanded disability status scale — also favored Ultomiris. However, group differences failed to reach statistical significance.
Safety-related findings were generally consistent with those reported in previous Soliris trials in NMOSD patients and Ultomiris trials for other approved indications.
After completing CHAMPION-NMOSD’s primary treatment period — which ended in March 2022 — 56 participants chose to enroll in the study’s long-term extension part, in which they would continue to receive Ultomiris for as long as two additional years.
Ongoing trial
Alexion also is testing Ultomiris in children and adolescents with NMOSD in an international Phase 2/3 trial (NCT05346354). A total of 12 participants, ages 2-17, with anti-AQP4 antibodies, are receiving Ultomiris at an initial loading dose, followed by a maintenance dose two weeks later, and every four or eight weeks thereafter, depending on weight.
Treatment will be given for 50 weeks, after which patients may choose to enter the trial’s extension period and continue to receive Ultomiris for up to two more years.
The study’s main goals are to assess relapse rates and the time to a first relapse. Secondary measures include changes in disability levels, walking skills, vision, and blood levels of Ultomiris and the C5 protein. Top-line data is expected by March 2026, and the trial is set to conclude in 2028.
Common side effects of Ultomiris
The most common side effects associated with Ultomiris in NMOSD clinical trials include:
- COVID-19 infection
- headache
- back pain
- joint pain
- urinary tract infection.
Infections
Ultomiris carries a boxed warning noting that it increases the risk of serious meningococcal infections, which can be life-threatening or fatal if not recognized and treated quickly. Patients should complete or update their meningococcal vaccination at least two weeks before the first dose of Ultomiris, according to current vaccination guidelines for patients on complement-blocking therapies.
If the risk of delaying Ultomiris treatment clearly outweighs the risk of meningococcal infection in a patient without updated vaccination, the person should receive a preventive course of antibiotics before starting Ultomiris. The vaccine should then be given as soon as possible.
Meningococcal infections can occur even with vaccination, so patients should be closely monitored for early signs and symptoms of such infections. These may include fever, headache, stiff neck, nausea and vomiting, confusion, and photophobia, in which the eyes are more sensitive to light.
In addition to meningococcal infections, treatment with Ultomiris also increases the risk of other types of infections, especially those from bacteria that are normally fought off by the complement cascade.
Infusion reactions
In the CHAMPION-NMOSD trial, 7% of NMOSD patients on Ultomiris experienced infusion-related reactions, or side effects that appear during or shortly after the infusion.
Such infusion-related reactions may include lower back pain, abdominal pain, muscle spasms, changes in blood pressure, chills or shivering, limb discomfort, allergic reactions, a bad taste in the mouth, and drowsiness.
Generally, experiencing infusion-related reactions does not require patients to stop taking Ultomiris. However, if patients show signs of cardiovascular or breathing problems, the infusion should be interrupted and appropriate supportive care should be given.
Use in pregnancy and breastfeeding
No solid data are available on the use of Ultomiris during pregnancy or breastfeeding. Animal studies have suggested that the therapy may cause harm to a developing fetus when used during pregnancy. Thus, patients who are pregnant or wish to become pregnant while using Ultomiris should discuss the potential risks and benefits of treatment with their healthcare team.
It remains unclear whether Ultomiris can be found in human breast milk, or if it has effects on nursing infants or on milk production. However, due to the potential for adverse reactions in a nursing child exposed to the medication, the FDA recommends that patients should not breastfeed while taking Ultomiris and for at least eight months after the last treatment dose.
Alexion is preparing an observational study (NCT06312644) to learn more about the safety of Ultomiris when administered during pregnancy and/or breastfeeding. The study will enroll up to 300 women with NMOSD or other diseases for which the therapy is approved.
Neuromyelitis News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Soliris reduces relapse rates in AQP4-positive NMOSD patients: Study
- The pain of NMOSD nerve sensitivity means more than just a cold shoulder
- Most adults with NMOSD use biologic therapies: Registry study
- Every time I turn to Dr. Google, I get an immune system mystery
- Conventional NMOSD treatment effective in double-negative cases
- Grieving how NMOSD has left me uncomfortable in my own skin
- Enspryng outperforms standard immunosuppressants in NMOSD study
- Slow and steady: I’m ditching big resolutions and easing into a new year
- Late-onset NMOSD responds better to newer, highly effective therapies
- New biomarker may help gauge disease severity in NMOSD
Related articles